Ò»¡¢Principle:
By applying the principle of ion exchange, high efficiency Dow Chemical patented ion exchange resin, with the pump constant flow of solvent (eluent) of the pump, the conductance of high sensitivity, etc. the analysis procedure is recorder, (pictured), constant flow pump and pump feeding by solvent, will be injected by the injection port. Body, push into the separation tube (Separation column), in the tube of the ionic exchange resin after separation, and then be pushed into the tube (Suppressor, Column) inhibited this solvent ion suppressed tube adsorption, does not affect the conductivity meter detection, and only the analysis of ions are developed into electric conductivity meter, the order and time of the entry (Retention time) to qualitatively, by the size of conductivity to quantitative, which are based on the peak (peaks) pattern, appear in the register . In the suppression tube, the reaction equation is as follows:
(1)2R£H++Na2CO3(eluent)¡ú2R£Na+H2CO3(Low conductivity)
R£H++NaHCO3(eluent)¡úR£Na+H2CO3
(2)R£H+Cation£A(sample)¡úR£Cation+H£A
A schematic diagram of ion tube column separation
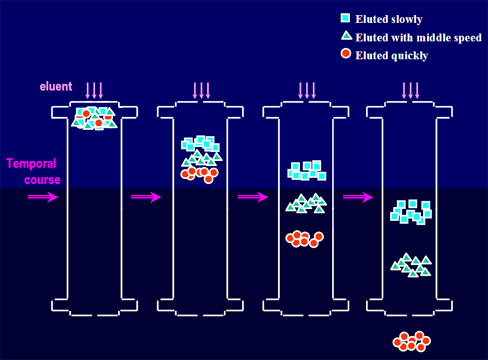
Figure 1. source of schematic data for ion tube column separation:Dionex
¶þ¡¢Basic structure of instrument
A.Pump
B.Separation tube column
B-1.The pH value of the anionic analysis is about 8~12, while the cation analysis is 2~5.
B-2.Resin separation column filling for low volume of organic anion exchange resin, strong acid and alkali resistant. Low capacity can prolong the life of tube column, and the inhibition effect is better.
C.Inhibition column: generally high capacity exchange resin, its effect:
• Reducing the conductivity of the extract
• Increase the degree of dissociation of ions
D.detector
D-1In general, the detector is a conductance meter
D-2The heavy metal analysis is not easy to be measured by the conductance meter, and the heavy metal ions can be formed by the chromogenic agent to form the wrong ion and then be measured by the UV/Vis detector.
E.Data processing system
Schematic diagram of ion chromatography system
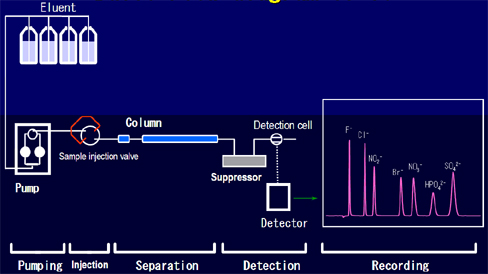
ͼ2. The source of the schematic data of the ion chromatography system:Dionex
Èý¡¢Applicable range of analysis:
The ions that can be analyzed by ion chromatography almost cover all the cations and anions. In some analyses, they replace A. A., Emission, GC, NMR, Ion Meter and so on. At present, the ions can be analyzed: halogen, organic acid, organic salt, phosphate, amine and so on.
ËÄ¡¢Characteristics:
1.The ion between ions does not interfere with the correctness of detection.
2.A variety of ions can be analyzed at the same time, and qualitative and quantitative analysis can be done.
3.High sensitivity (below 10ppb), suitable for a wide range of concentration (0.001~103ppm).
4.The analysis is fast, and each ion is not more than two minutes.
5.The use of solvent as a general inorganic solvent, cost economy.
Îå¡¢Summary:
The ion chromatography is separated by the different affinity between the active phase (the extract liquid) and the static phase (the ion exchange resin in the tube column). In general, the detector (Detector) is conductance meter. The measured conductance signals of each component can get the peak area after the circuit is integrated, and the area is used as a quantitative. The principle of a tube is to use a strong affinity resin. The ion is not easily taken out, and the retention time is long. The affinity is weak, the anion is easy to maintain the original ion state and the ion retention time is short. Eluent is generally strong electrolyte, high concentration and volume, resulting in the background value is very high, the signal to noise ratio (S/N) becomes small, often covering for analysis of ion signal, therefore in the eluent through the separation column after a suppression column, will put in strong electrolyte solution at change the weak electrolyte, and the original desire analysis by ion salts into acid or alkali and enhance its conductivity.


